Understanding the Drying and Cracking Mechanism of Lithium-ion Battery Anode Electrode Pieces
The drying and cracking of the lithium-ion battery anode electrode piece is a prevalent issue, primarily occurring when the dispersion coating is dried on a non-porous rigid substrate such as a current collector. As the solvent evaporates, the coating shrinks, generating stress due to the substrate's rigidity. When this stress exceeds the binding force between particles, cracks appear.
Mechanism of Drying and Cracking in the Lithium-ion Battery Anode Electrode Piece
The central region's cracking of the battery anode piece is often attributed to insufficient local capillary cohesion and crack development driven by solvent evaporation. Edge area cracking, however, is affected by factors such as horizontal flow, uneven solute concentration, and transverse shrinkage caused by binders.
Crack Group Dynamics in the Central Body of Lithium-ion Battery Anode Electrode Pieces
During early drying, air infiltrates the particle network, forming a meniscus at the gas-liquid interface. This capillary action promotes particle aggregation, leading to gaps that become crack sources. As drying progresses, internal solvents evaporate, widening these cracks, especially in graphite and carbon black coatings with high elastic moduli.
Edge Area Cracking in Lithium-ion Battery Anode Electrode Pieces
Cracking in edge areas results from the lateral movement of components like carbon black and binders during drying. Differences in solute concentration create varying capillary forces across the wet-dry interface, intensifying the risk of cracking due to tensile stresses.
Factors Affecting the Drying and Cracking of Lithium-ion Battery Anode Electrode Pieces
Driving Force and Stress Factors
Capillary pressure is a primary factor in cracking, developing from solvent evaporation that forms the inter-particle meniscus. The generated tensile stress can exceed the lithium-ion battery anode electrode piece's tolerance, leading to cracking.
Material and Thickness Considerations
Particle size, binder type, and coating thickness significantly influence crack formation. Smaller particle sizes reduce cracking risk, while different binders affect total material stress and crack propagation. Moreover, variations in coating thickness directly affect crack morphology and density.
Current Collector Material Influence
The substrate material's characteristics, such as wettability and stiffness, affect crack development by altering coating and substrate interactions. Hydrophobic substrates and materials with low moduli of elasticity help mitigate cracking in lithium-ion battery anode electrode pieces.
Impact of Solvent Evaporation Rate
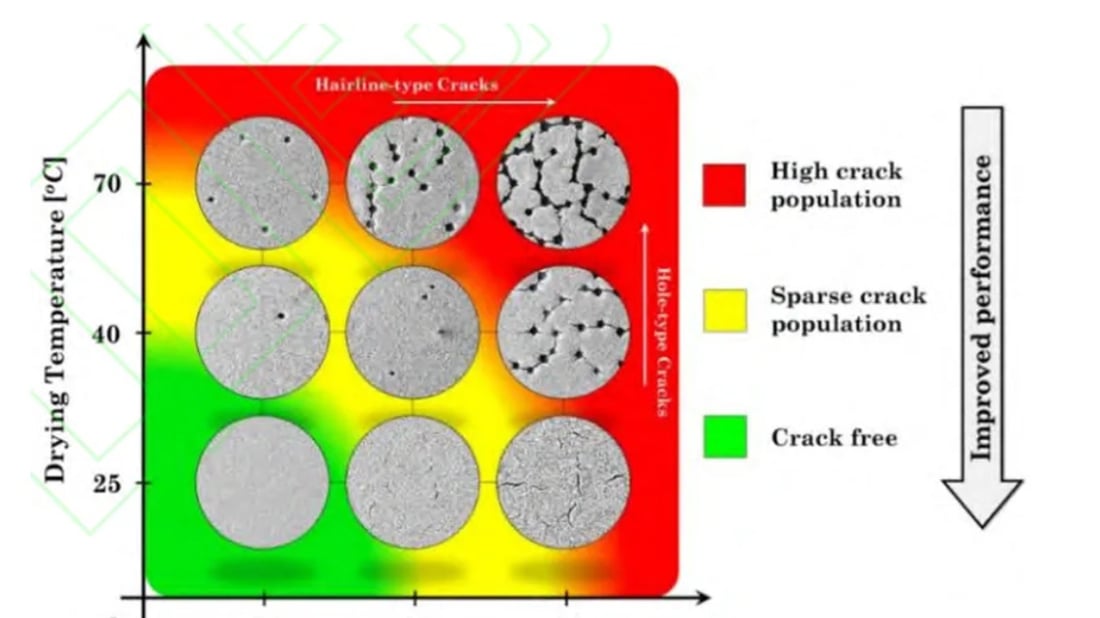
A controlled drying environment, in terms of temperature and humidity, stabilizes the coating of the lithium-ion battery anode electrode piece. High temperatures and low humidity increase internal stress, while coating thickness and area mass load impact evaporation uniformity.
Concluding Thoughts
To address cracking during the drying process, managing intrinsic coating characteristics and boundary conditions is vital. Proper selection of coating materials, controlling coating thickness, optimizing current collector material choice, and regulating solvent evaporation rates are crucial for effective crack control in lithium-ion battery anode electrode pieces.