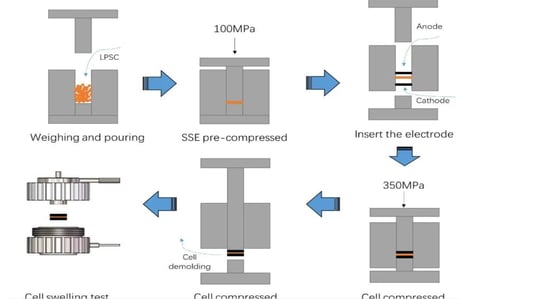
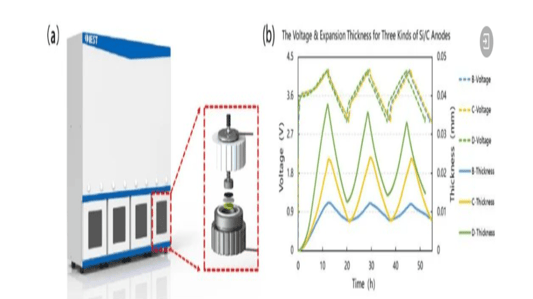
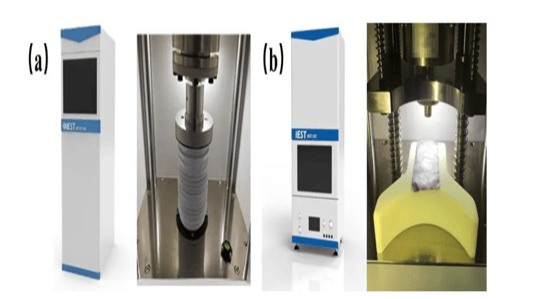
An In-Depth Look at Sulfide all solid state battery ExpansionThe Rise of Solid State BatteriesWith the advancements and limitations of liquid batteries becoming apparent, the focus has shifted towards the future of lithium batteries: sulfide all solid state batteries. These batteries boast solid electrolytes that offer impressive mechanical properties and the ability to prevent short circuits caused by lithium dendrites. Solid electrolytes also provide high chemical and thermal stability, reducing safety risks at elevated temperatures.Sulfide All Solid State Battery: The Technological FrontierThe sulfide all solid state battery is part of a rapidly developing industry supported by government initiatives. The key technological pathways in this domain include oxides, polymers, and sulfides/halides. Within the landscape of sulfide all solid state batteries, each component—positive electrode, negative electrode, and electrolyte—consists of solid powders compacted under high-pressure conditions.Experimental Approach to Sulfide All Solid State Battery ExpansionRecent insights into the expansion behavior of sulfide all solid state batteries have been provided by Yuanneng Technology's Solid State Electrolyte Testing System (SEMS) and the Silicon Negative Expansion Rapid Screening System (RSS). These systems evaluate how particles in sulfide all solid state batteries expand and contract more significantly than their liquid counterparts during charging and discharging cycles.Detailed Battery Assembly and TestingThe experiment focused on a sulfide all solid state battery button-type model. The electrolyte layer was pre-pressed and inspected for defects before assembly. The cell was then fully pressurized using 350MPa to ensure integrity. Subsequent testing with the RSS showed variable expansion during the charging and discharging cycles, providing critical data on sulfide all solid state battery behavior.Findings and Implications for Sulfide All Solid State Battery DevelopmentThe experimental results highlighted a consistent pattern: sulfide all solid state batteries experience significant but ultimately stabilizing expansion. Specifically, the capacity of these batteries declined sharply during initial cycles due to irreversible expansion, showcasing the challenges while emphasizing the potential of the sulfide all solid state battery systems.Conclusion: The Future of Sulfide All Solid State BatteriesThis study underscores the importance of continued research on sulfide all solid state batteries, particularly in optimizing pressure conditions to minimize expansion and capacity loss. Yuanneng Technology's testing systems prove instrumental in this ongoing evaluation, fostering faster advancements and the evolution of robust sulfide all solid state battery technologies.Quote Inquirycontact us