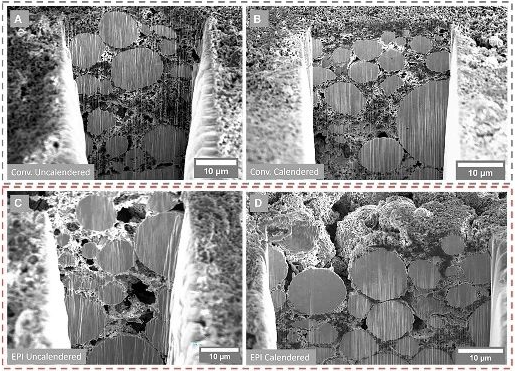
Liquid lithium batteries have become the mainstream of the industry, but there are still some problems in the application side that need to be solved. Whittingham first proposed and began research on lithium-ion batteries in the 1970s, and then in the 1990s, SONY invented a commercial liquid lithium battery. At present, liquid lithium batteries have become the mainstream of the industry with their advantages of high energy density, fast charging speed, long life and no pollution. However, at the same time, there are still some problems restricting the future development and application of liquid batteries.1) Safety issues: The electrolyte and diaphragm in the liquid lithium battery structure may cause safety issues. The problem of lithium dendrites: lithium ions are removed between positive and negative electrodes to achieve the charge and discharge of lithium batteries. However, when there are some abnormal conditions that make lithium ions cannot be normally removed, lithium dendrites may be formed. The accumulation of lithium dendrites to a certain extent may puncture the battery diaphragm, short-circuit the positive and negative electrodes, and then cause fire and other safety problems. Electrolyte problem: liquid lithium battery electrolyte commonly used lithium salt dissolved in volatile, combustible organic solvents, the safe operating temperature is generally lower than 80 ° C, so when the battery temperature is too high, combustion and explosion may occur. Problems with diaphragms: The ordinary diaphragms used in batteries have low thermal conductivity, which can reduce the rate of heat dissipation in lithium-ion batteries.2) Energy density problem: the current energy density ceiling of liquid lithium batteries is 300Wh/kg, and solid lithium batteries can reach a higher energy density by updating the material system, which can reach 700Wh/kg or more. In recent years, solid-state battery technology has rapidly improved and is expected to become a new generation of lithium battery technology. As early as 1830s, Faraday first discovered the remarkable conduction properties of heated solid silver sulfide and PbF2. Then in the 1960s, the solid electrolyte ushered in a turning point in development, and began to try to add the solid electrolyte to the battery. Subsequently, POE, sodium superionic conductor, hydride, LiPON, sulfide, antiperovskite and other solid electrolyte systems appeared successively. Along with the development of LiPON, the first thin-film battery was introduced in the 1990s. After entering the 21st century, the related research of solid electrolyte has been further developed. With the advantages of structural design and physical characteristics, solid-state batteries can naturally avoid the safety and energy density problems of liquid lithium batteries. Therefore, solid-state batteries have developed rapidly in recent years and are expected to become the next generation of lithium battery technolog Revolutionizing Energy Storage: The Rise of Solid-State BatteriesSolid-state batteries use solid-state electrolytes to replace the electrolyte and diaphragm in liquid lithium batteries. Solid-state batteries are a new technology that uses solid-state electrolytes to replace the electrolyte and diaphragm in liquid lithium batteries. The main material of the traditional liquid battery is the positive electrode, the negative electrode, the diaphragm and the electrolyte. In the process of charging and discharging, the electrolyte supplies some active lithium ions as conductive ions on the one hand, and provides ion channels so that lithium ions can move freely. The role of the diaphragm is mainly to make the normal passage of electrolyte ions, and to avoid internal short circuit caused by positive and negative electrode contact. In solid state batteries, because the physical form of solid state electrolysis can naturally isolate the positive and negative electrodes, the main material in solid state batteries is positive, negative and solid electrolyte, and the diaphragm is not necessary.Challenges and Potential of Solid-State Batteries: Enhancing Safety and Energy Density.Solid-state batteries can overcome the shortcomings of lithium-liquid batteries and achieve further breakthroughs in safety and energy density. In terms of safety, solid state batteries use non-combustible solid electrolyte, with no corrosion, no volatilization, no leakage, can inhibit the formation of lithium dendrites, natural isolation of positive and negative poles, and so on, with high safety. In terms of energy density, solid-state batteries can use materials such as lithium metal as a negative electrode instead of graphite, thereby increasing the energy density of the battery. However, solid-state batteries still face problems in material performance and cost, and the current large-scale industrialization is still facing certain challenges. The electrolyte material makes fast-charging weaker than liquid batteries. Compared with liquid batteries, solid-state batteries use solid electrolytes, so the ionic conductivity is relatively low, which makes the fast charging ability weaker than liquid batteries. Poor interface contact between solid electrolyte and electrode. In a liquid battery, the contact between the liquid electrolyte and the electrode is good, but in a solid battery, there may be a gap between the solid electrolyte and the electrode, and the solid-solid contact interface leads to poor stability. Solid-state batteries are expensive. According to the analysis of South Korean market research institution SNE, the high cost of solid-state batteries is mainly affected by two factors, one is the high cost of raw materials, including the high cost of lithium sulfur compounds; Second, the manufacturing cost is high, which is due to the high requirements of solid state battery production on raw material purity and synthetic environment, making the manufacturing cost higher.Key Differences Between All-Solid-State, Semi-Solid-State, and Liquid BatteriesThe all-solid-state battery differs greatly from the existing liquid battery system, while the semi-solid-state battery differs little from the liquid battery system. A semi-solid battery is a lithium battery that contains both solid and liquid electrolytes. In contrast, in addition to electrolytes, the main difference between liquid batteries and solid batteries is reflected in the negative electrode of the liquid battery graphite or silicon carbon, while the negative electrode of the solid battery is metal lithium. However, there is little difference between the semi-solid battery and the existing liquid battery system, some materials can be common with liquid batteries, including the need for diaphragms, etc., and the manufacturing process is also mostly overlapping.Evolution of Battery Technology: From Liquid to Solid State There are still difficulties in the current mass production of all-solid-state batteries, and semi-solid-state batteries may become transition products. At present, due to the mass production of all-solid-state batteries is still facing a series of problems, so the development and penetration of solid-state battery technology in the future will take some time. The semi-solid battery due to the small changes in the material system of the battery, the manufacturing process and technology can also follow the technical route of the liquid battery, so it may become a transition product from the liquid battery to the all-solid battery in the short term. It is expected that the technology of lithium batteries in the future will follow the development path of liquid lithium-ion batteries - semi-solid batteries - all-solid batteries. It is expected that the technological iteration path of solid-state batteries is electrolyte-negative-positive. From the perspective of research and development, solid-state batteries have three stages, the first generation is to replace the traditional electrolyte and diaphragm with solid electrolyte; The second generation uses lithium metal as a negative electrode material to increase the energy density; The third generation is based on the second generation, and further replaces the positive electrode material with a higher energy density material to further improve the energy density of the battery. Solid state batteries gradually increase energy density through battery material scheme iteration. Taking Solid power's product iteration route as an example, its first solid-state battery product uses "electrolyte + silicon anode +NCM811", the energy density can reach 390Wh/kg, and the cycle life can reach more than 1000 times; Its second product iterates the negative electrode technology, using "electrolysis + lithium metal negative electrode +NCM811", the energy density can reach 440Wh/kg; The third product is based on the second product to develop the next generation of positive electrode materials, using "electrolyte + lithium metal negative + next generation positive electrode", the energy density can be further increased to 560Wh/kg.
11. June, 2024





![What is SBR (Styrene butadiene Rubber)? [Lithium ion battery materials]](https://cdn1.funpinpin.com/cdn-cgi/image/w=533,dp=2,format=auto/social-media-manager-headphoto/6b12a10310/202406/istockphoto-1219511466-612x612.jpg)