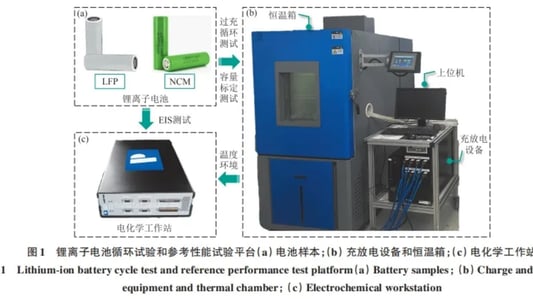
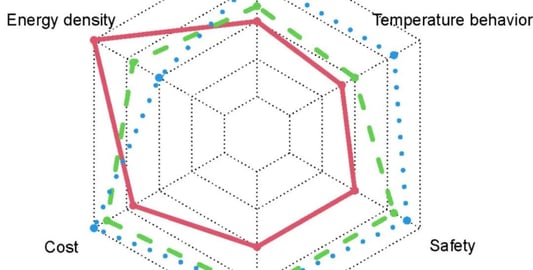
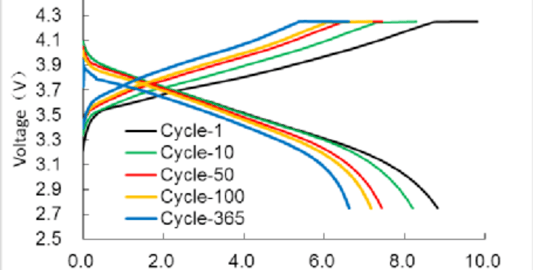
lithium battery degradation Under Micro Electric AbuseThe widespread use of lithium-ion batteries in clean transportation and large-scale energy storage has raised concerns about lithium battery degradation due to manufacturing inconsistencies and variable decay rates. One prevalent issue is slight overcharging during battery operation, often leading to electrical abuse conditions.Impact on Performance: Comparing Battery TypesThis analysis focuses on lithium battery degradation in two types: lithium iron phosphate and nickel cobalt manganese ternary lithium-ion batteries. Through cycle tests—normal cycle aging and micro overcharge cycle aging—the study identifies the differences in lithium battery degradation characteristics under these conditions.Results: Ternary vs. Lithium Iron Phosphate BatteriesInterestingly, for lithium iron phosphate batteries, lithium battery degradation presented minimal differences between micro overcharge and normal cycling. Conversely, ternary batteries exhibited a considerably higher lithium battery degradation rate under micro overcharge conditions compared to normal cycles.Reasons Behind DegradationElectrochemical impedance tests and equivalent circuit model analysis pointed to substantial lithium battery degradation in ternary batteries. This was attributed to increased equivalent resistance at the solid electrolyte interface and load transfer resistance, intensifying lithium inventory loss. Conversely, lithium iron phosphate batteries demonstrated limited lithium battery degradation due to stable resistance metrics.Conclusion: Resistance Properties and Lithium Battery DegradationThe comparative analysis of lithium battery degradation highlights that lithium iron phosphate batteries are more resilient to micro electric abuse conditions. Their resistance properties remain stable, minimizing lithium inventory loss and hence reducing lithium battery degradation amplitude. This resilience offers significant advantages in maintaining performance consistency across cycles.Quote Inquirycontact us