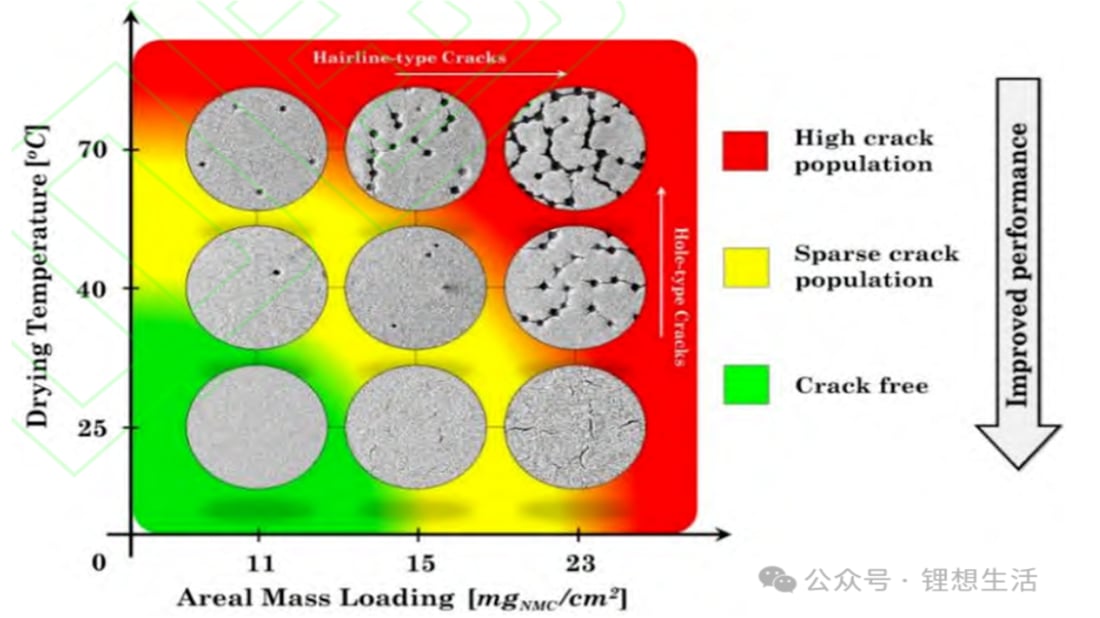
Drying and Cracking Mechanisms in Lithium-Ion Battery Electrodes
Cracking in lithium-ion battery electrodes is a well-documented issue during the drying phase. When a dispersed layer of coating is applied to a non-porous, rigid substrate – such as a current collector – and subsequently dried, the solvent evaporates from the substrate's surface. This evaporation leads to the downward deposition of particles, causing overall shrinkage of the coating. These constraints generate stresses on the polarizer due to the substrate’s rigidity. Once these stresses exceed the bonding strength between particles, cracking occurs to release them.
Role of Capillary Pressure in Lithium-Ion Battery Electrode Cracking
Capillary pressure is widely regarded as the main contributor to drying cracks in lithium-ion battery electrodes. Different areas of the electrode coating exhibit variations in cracking mechanisms during the drying and film-forming process. In the central region, cracking usually results from inadequate local capillary cohesion and solvent evaporation-induced crack propagation. On the other hand, the peripheral regions are more affected by horizontal flow, uneven solute concentration, and binder-induced lateral shrinkage.
Lithium-Ion Battery Electrode Central Area Cracking
In the initial drying stages of lithium-ion battery electrodes, air permeates through the particle network, forming meniscus between solute particles, which promotes particle aggregation. These meniscus surfaces generate capillary action, creating a cohesive force. Weaker capillary cohesion due to larger particle gaps can become crack initiation points as the solvent evaporates. Further solvent evaporation and diffusion through existing cracks cause them to widen, while the high elastic modulus of core particles like graphite prevents stress relief through deformation, intensifying internal stress and cracking susceptibility.
Peripheral Edge Cracking in Lithium-Ion Battery Coatings
In lithium-ion battery coatings, horizontal flow during drying allows carbon black, CMC, and SBR to move freely, accumulating at dry-wet interfaces and creating thicker regions. Increased capillary force develops in regions with small particle aggregation at these interfaces, leading to higher tensile stress and crack risk as the solute concentration varies. The accumulation of adhesive near dry-wet interfaces causes significant lateral shrinkage, resulting in substantial residual thermal stress that can lead to cracking.
Critical Factors in Lithium-Ion Battery Electrode Cracking
The driving force behind cracking in lithium-ion battery electrodes during drying is solvent evaporation. This process leads to the formation of meniscus between particles, triggering capillary pressure, represented by the surface tension at the liquid-gas interface, the contact angle, and the curvature radius of the meniscus. Under capillary pressure, the particle network begins to shrink, causing volume shrinkage and subsequent tensile stress inside the coating once constrained by the substrate. The coating cracks when this tensile stress exceeds the coating's bearing limit.
To analyze these defects, two critical boundary conditions are proposed: critical crack-free internal stress and critical crack-free film thickness. These provide theoretical insights for manufacturing crack-free electrode sheets in lithium-ion batteries. The critical crack-free internal stress refers to the maximum internal stress that the coating can withstand without cracking, while the critical crack-free film thickness is the maximum safe thickness for crack-free coatings, influenced by material characteristics, solvent evaporation rates, drying conditions, and substrate properties.