Lithium Battery Expansion and Graphite Electrode Dynamics
The Challenge of Expansion in Lithium Batteries
Expansion is a significant issue in lithium batteries, especially for large aluminum shell and flexible pouch types. Variations in thickness and internal stress can negatively affect battery performance, impacting its service life and reliability.
Core Causes of Battery Expansion
The swelling of lithium batteries is primarily due to two factors. First, the thickness variation in battery electrodes, especially graphite electrodes, plays a critical role. Second, electrolyte decomposition produces gases that lead to expansion. Research pinpoints electrolyte decomposition as the main instigator, with variations arising from impurities or a narrow electrochemical window.
Graphite Electrode and Electrode Thickness Variation
For graphite electrodes, changes in thickness occur due to several reasons. Compaction processes during production can lead to a rebound effect, influenced by compaction density and the elastic modulus of the adhesive. Additionally, impregnation with electrolyte can swell the electrode.
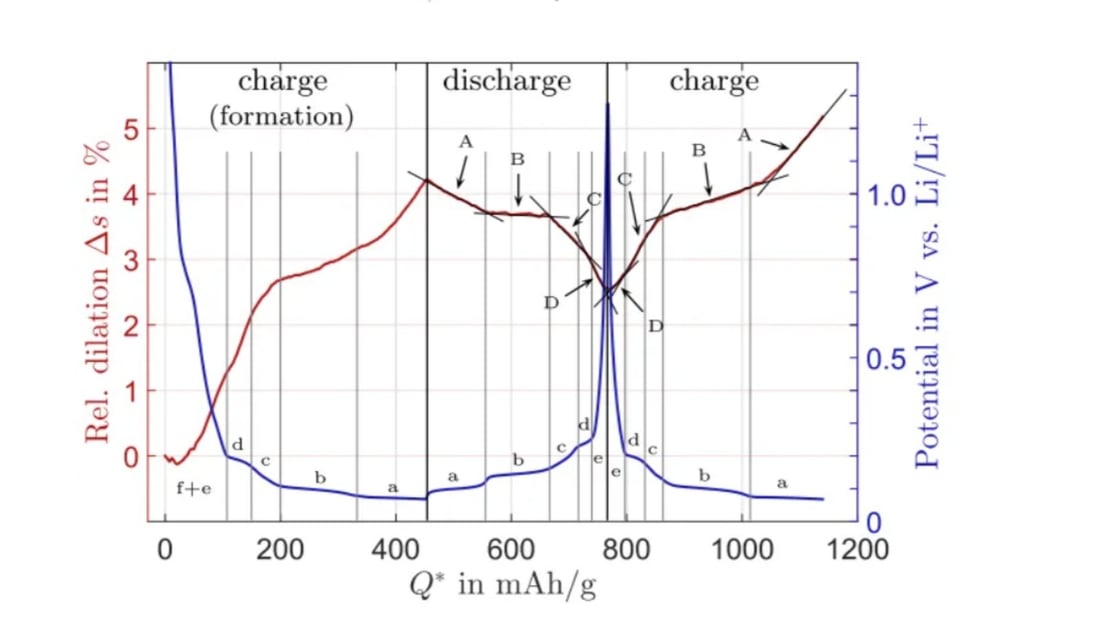
Detailed Analysis of Graphite Electrode Lithiation
This article delves into the mechanisms of graphite electrode lithiation, crucial for lithium-ion battery performance. Upon discharge and lithiation, lithium ions are inserted between graphite layers, causing electrode expansion.
Phases of Graphite Electrode Lithiation
During the lithiation of graphite electrodes, several stages and phases emerge. Initially, as lithium atoms embed between layers, various LixC6 compounds form. The graphite transitions from the 2H phase to LiC12 at about a 50% SOC and finally to LiC6 when fully lithiated, achieving a theoretical capacity of 372mAh/g.
Impact on Battery Design and Longevity
Understanding graphite electrode behavior is necessary for optimizing battery design, stability, and reliability. The expansion from lithiation involves interlayer spacing increases, critical for maintaining battery integrity.