aa lithium ion battery Performance and Cycle Life Analysis
Understanding Capacity Decay in AA Lithium Ion Batteries
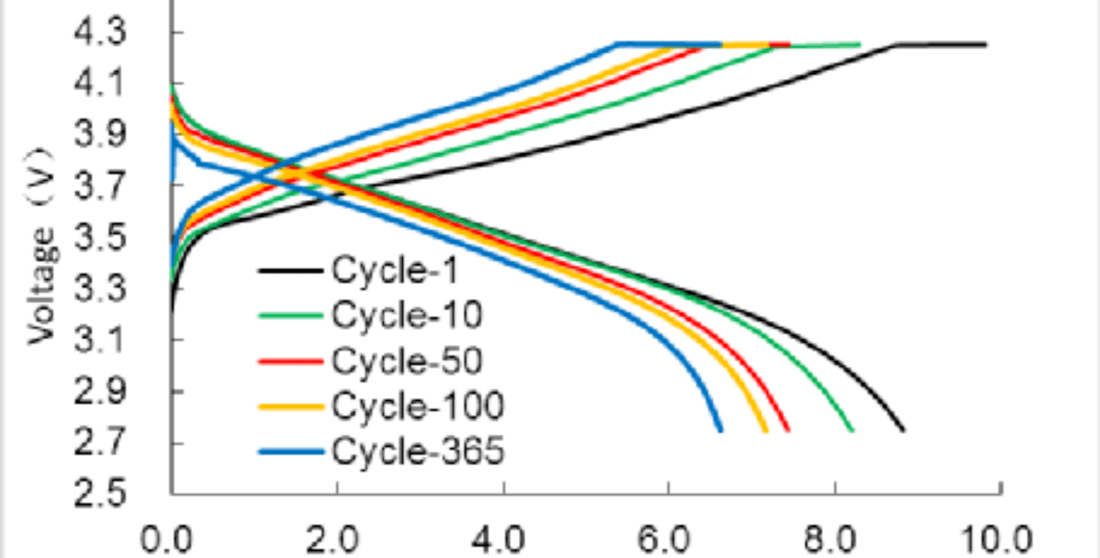
The use of AA lithium ion batteries is widespread, but over time, their actual available capacity diminishes. This inevitable capacity decay is linked to side reactions that consume lithium ions, affecting the battery's overall balance and performance. Each charging and discharging cycle contributes to this decay, underscoring the importance of understanding the cycle life of AA lithium ion batteries.
Factors Affecting Cycle Life
One major factor is the persistent decrease in the number of lithium ions taking part in energy transfer, although the total lithium in the AA lithium ion battery remains unchanged. These ions, when trapped or blocked, fail to participate actively in charge and discharge processes. The following outlines specific issues leading to decreased cycle life.
Lithium Deposition on Negative Electrode
During operation, lithium metal can deposit on the negative electrode of the AA lithium ion battery. If lithium ions fail to integrate effectively, they form metallic lithium, thus losing efficacy in successive cycles. This problem often arises from overcharging or inadequate negative electrode material.
Decomposition of Positive Electrode Material
Over time, the metal oxides in the positive electrode of the AA lithium ion battery can decompose, generating inert substances and gases. This decomposition disrupts electrode balance and results in irreversible capacity loss.
SEI Film Formation and Breakdown
Initially, a solid electrolyte interface (SEI) film forms on the carbon negative electrode, consuming lithium ions. As cycling continues, the SEI film fractures, causing new unreacted surfaces to react with the electrolyte. This continuous process impacts lithium ion availability, affecting battery capacity.
Electrolyte Loss
Repeated cycling leads to electrolyte decomposition in the AA lithium ion battery, reducing the amount needed for positive and negative electrode infiltration, thereby limiting charge and discharge efficacy. Additional reactions if water is present can damage SEI film further reducing battery life.
Membrane Blocking or Damage
Deterioration of the diaphragm increases ohmic resistance and blocks battery channels, preventing AA lithium ion battery capacity from fully recharging, thus shortening its service life.
Electrode Material Detachment
The active materials of electrodes are bound to substrates, but long-term usage can weaken this bond, causing particles to disengage and enter the electrolyte. This reduces the active material available for future cycles, hastening battery capacity decline.
Standard Testing for Battery Lifespan
Testing methods for AA lithium ion batteries generally include continuous charging-discharging cycles, which are time-consuming. Standards indicate specific requirements and testing methods to assess cycle life effectively, as detailed in existing Chinese standards.