Lithium-ion battery (LIBs) has become one of the widely used electrochemical energy storage systems due to its high energy density, high operating voltage and no memory effect, and its commonly used graphite negative electrode is difficult to fully meet the increasing market demand due to its relatively low capacity (372 mAh g-1). Over the past few decades, researchers have proposed a variety of new anode materials, which generally exhibit the advantages of ideal potential range, higher capacity, excellent magnification performance, and long cycle life, but have the disadvantage of large initial active lithium loss (ALL). Therefore, how to eliminate ALL before full battery assembly is critical to achieving high-performance LIBs. In the course of development in recent years, new anode materials for the next generation of LIBs have gradually begun to be commercialized, so the research of pre-lithium technology, which is crucial to the elimination of ALL, has become an important research direction.
Causes of loss of high initial active lithium in negative electrode
The high initial ALL of the negative electrode occurs in the first few cycles and the coulomb efficiency is low (CE < 100%), which indicates that some Li+ remains in the negative electrode, resulting in a decrease in the amount of Li+ that can be cycled in the LIBs. When matched with the positive electrode, the reduced recyclable Li+ will inevitably lead to a reduction in the energy density of the entire battery. Figure 1 shows the typical intercalation/insertion, conversion and alloying lithium storage mechanisms of negative electrode materials, which mainly exhibit relatively low potential and much higher capacity than commercial graphite and Li4Ti5O12, but the first loop coulomb efficiency of these materials is usually less than 80%, resulting in a low coulomb efficiency mechanism. The causes of initial negative ALL can usually be divided into the formation of SEI, the loss of active material and the appearance of dead lithium.
Effect of negative active lithium loss
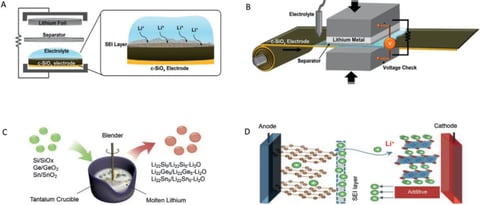
In practical applications of LIBs, some of the recyclable Li+ is consumed to form SEI on the negative surface, resulting in a lower first turn of CE, which in turn leads to a rapid capacity decay of the battery. As shown in Figure 2, the reversible capacity of the electrode is not reduced during this process, and when additional lithium sources are added to the system, the specific capacity of the battery will be restored to the ideal situation. The introduction of additional lithium sources will offset the specific energy gain brought by the pre-embedded lithium, and the effect of higher initial ALL on the specific capacity loss of the whole battery can be elaborated through theoretical calculation and analysis, and the specific energy based on the total mass of the negative electrode, the positive electrode and the lithium source can be obtained
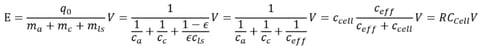
Figure shows the effect of different additional lithium sources on contrast energy. The specific capacity function of R with respect to the lithium source (cls) is shown for different negative lithium sources with initial CE of 50%, 70% and 90%, respectively. It can be seen that with the increase of cls, the R factor increases, while the decrease of CE will lead to a lower R factor. It can also be seen that when cls is greater than cc, it is necessary to use lithium sources to effectively improve the energy density. The analysis of these results can add more detailed parameters for different systems.
A lithium source is added to the negative electrode
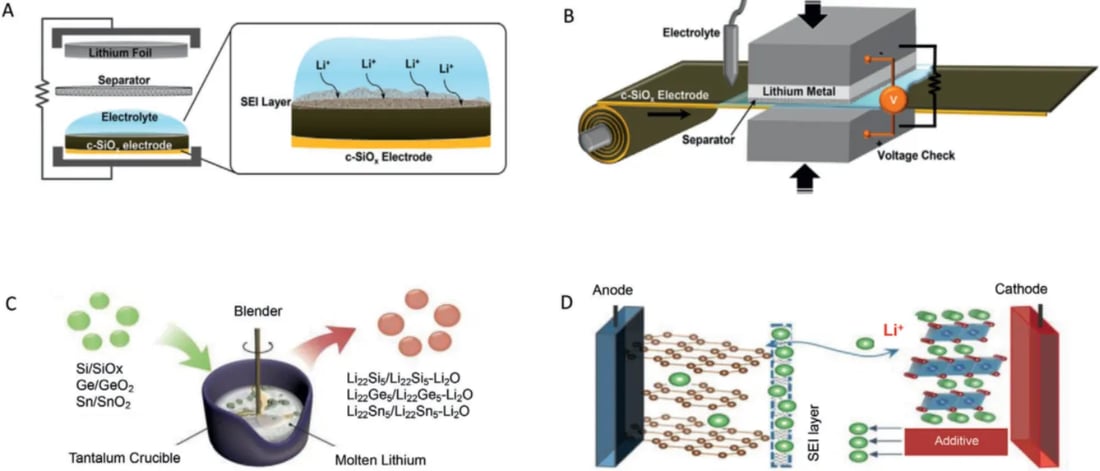
Initial ALL is caused by irreversible electrochemical processes on the negative electrode, so the most direct strategy for eliminating initial ALL is to prepare the pre-embedded lithium negative electrode by electrochemical and/or chemical strategies prior to pairing with the positive electrode. The positive electrode strategies can be divided into three categories: the half cell electrochemical method (HC-EM), the short circuit electrochemical method (SC-EM) and the chemical method (CM) as shown in the figure. After the negative electrode is pre-embedded with lithium, the initial ALL problems can be solved well, and the Coulomb efficiency of the entire battery first circle can be effectively improved.