Understanding Conductive Percolation Theory in Lithium Ion Batteries
Challenges with Lithium Ion Battery Electrode Plates
In lithium ion batteries, the positive electrode materials often exhibit poor electronic conductivity. To enhance the electronic conductivity in these essential battery components, it becomes crucial to incorporate conductive agents. These agents serve to facilitate the transfer of electrons and the collection of microcurrents between the active material particles and the current collector. By minimizing contact resistance, they substantially alleviate the polarization phenomenon common in lithium ion batteries.
The Role of Conductive Agents
Constructing a conductive network within the lithium ion battery electrode is influenced by the distribution and morphology of the conductive agents used. The percolation theory model excels in predicting and determining the likelihood of creating a continuous conductive network at certain concentrations. This theoretical model provides a profound understanding crucial for developing composite materials with superior conductivity attributes.
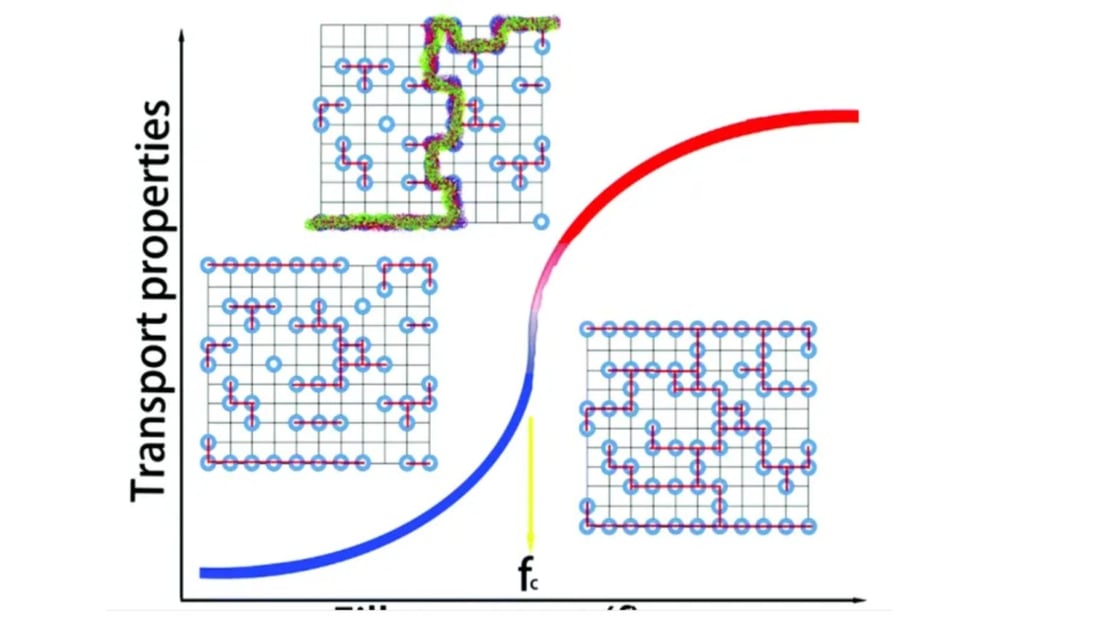
The Application of Percolation Theory
Initially introduced to examine physical phenomena in disordered systems, percolation theory now offers insights into heterogeneous multi-component materials' properties, including conductivity. In the realm of lithium ion batteries, as the filler content approaches the percolation threshold, there's a noticeable nonlinear transformation in the composite material's physical properties.
Optimizing Electrode Formulations for Lithium Ion Batteries
Leveraging percolation theory provides an avenue for optimizing lithium ion battery electrodes by maintaining adequate conductive agent content. This strategy ensures improved conductivity performance while preventing any adverse effects from excessive agent addition.
Various Conductive Agents and Their Impact
Conductive agents manifest in multiple forms that influence the conductivity of lithium ion battery electrodes. Zero-dimensional granular conductive agents distribute evenly, favoring local electron pathways but lacking in facilitating electron transport in the electrode's thickness direction.
Conversely, one-dimensional fibrous agents face challenges in forming close contacts with active materials due to their structure, compromising local electron conductivity. Nevertheless, their long-chain arrangements support extensive electron conduction across the electrode.
Two-dimensional sheet-like agents such as graphene provide exceptional conductivity through their ultra-thin architectures and "surface point" contacts, greatly benefiting the electronic conductivity of lithium ion batteries. By synergizing different conductive agent structures, one can establish a more comprehensive and efficient conductive network within lithium ion battery electrodes.