Understanding the Influence of Carbon gel phase on Lithium-Ion Diffusion
Introduction to Electrode Coating Composition
The carbon gel phase is crucial in the coating composition of lithium-ion battery electrodes, playing a role alongside pores, active material (AM), conductive agents, and binders. The active material facilitates lithium ion insertion and extraction, while the carbon gel phase disperses between active particles. The binder provides mechanical stability by binding solid particles, and conductive agents create electron transmission channels.
Role of Carbon Gel Phase in Lithium-Ion Transport
Pores filled with electrolyte aid in lithium ion transportation. Using 3D electrode micro modeling, this study simulates GITT to explore how the carbon gel phase affects the diffusion coefficient of lithium ions in NMC111 electrodes.
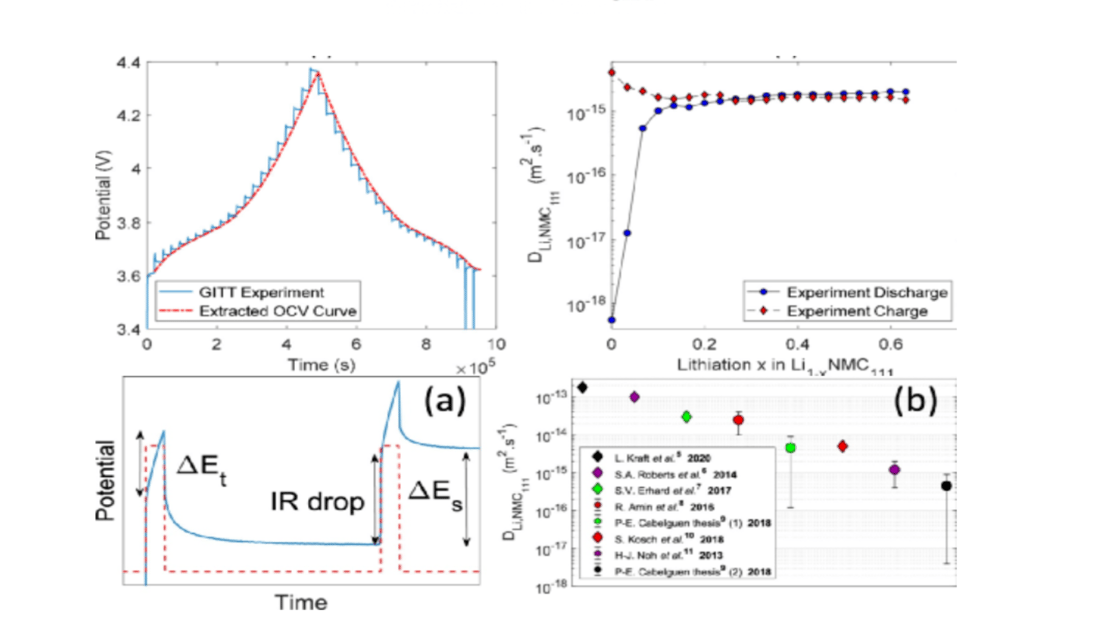
GITT: Galvanostatic Intermittent Titration Technique
GITT, a transient measurement method, involves constant current pulse relaxation cycles. By charging or discharging the NCM/Li half cell at a constant current of 0.1 C for 30 minutes before a 6-hour pause, researchers can infer reaction kinetics from the polarization data of electrodes.
Diffusion Coefficient and Carbon Gel Phase
The diffusion coefficient formula relates to active particle radius, pulse time, steady-state voltage changes, and total voltage change during the pulse period. This assumes one-dimensional lithium diffusion and neglects phase transitions and charge transfers between AM and the carbon gel phase. Small current application ensures consistent diffusion coefficient measurement.
Simulation of Electrochemical Performance through Microstructure Construction
Electrode microstructure is simulated using coarse-grained molecular dynamics (CGMD) to generate mesoscopic structures in electrode preparation. Carbon gel phase particles are simplified for computational efficiency before being imported into COMSOL software for performance predictions.
Impact of Carbon Gel Phase on Diffusion Coefficient and Simulation Results
Simulation considers three scenarios: blocking CBD which halts Li transport, partially open CBD allowing some diffusion, and fully open CBD enabling high Li transport. The carbon gel phase significantly affects diffusion, with fully open CBD showing higher lithium transport and diffusion than a blocked CBD.
Understanding the Difference in Diffusion Coefficient
Although the simulated OCV curves resemble experimental ones, they are lower in value. The microstructure of electrodes affects GITT-calculated diffusion coefficients, with uneven lithium concentration gradients influencing differences in observed D Li.
Conclusion: The Crucial Role of Carbon Gel Phase
The carbon gel phase exerts significant control over lithium transport and diffusion in electrodes. Greater blockage results in increased transport restriction and lower diffusion coefficients, underscoring the importance of microstructural design in electrode performance.