Effect of Carbon Colloidal Phase on 3D Electrode Microscopic Modeling
The lithium-ion battery electrode coating consists of a mixture involving the carbon colloidal phase CBD, intricately combined with pores, active material (AM), a conductive agent, and a binder. The active material acts as a vessel for lithium ion ingress and egress, while the carbon colloidal phase is distributed among active particles. The binder’s role is to amalgamate the solid particles, ensuring mechanical cohesion, whereas the conductive agents create pathways for electron movement. Pore-filled electrolytes serve lithium ion transport. This study examines the effect of carbon colloidal phase on the NMC111 lithium-ion diffusion coefficient through detailed 3D electrode microscopic modeling and simulations using the Galvanostatic Intermittent Titration Technique (GITT).
Understanding the GITT Principle
The Galvanostatic Intermittent Titration Technique (GITT) serves as a core measurement avenue. It involves a cycle of galvanostatic pulse and relaxation—where 'pulse' refers to short-term current application, and 'relaxation' denotes the null current phase. Typically, in a specific time frame t, a constant charge or discharge current I is applied, followed by a no-current phase to achieve balance before the subsequent pulse. This dual-phase procedure helps record voltage changes during current pulses and relaxations.
Application of GITT in Lithium-ion Analysis
By leveraging GITT, we deduce polarization data and subsequently infer reaction kinetics, often measuring ionic diffusion coefficients. The effect of carbon colloidal phase plays a pivotal role in interpreting these measurements. The derived diffusion coefficient D, considering certain assumptions, ideally applies to singular spherical particles. While transitioning to systems with multiple particles requires assumption adjustments such as constant particle surface area-to-volume ratio, one-dimensional lithium diffusion within the active particle, while ignoring complex charge or transition phases associated with AM and CBD.
Constructing Electrode Microstructures
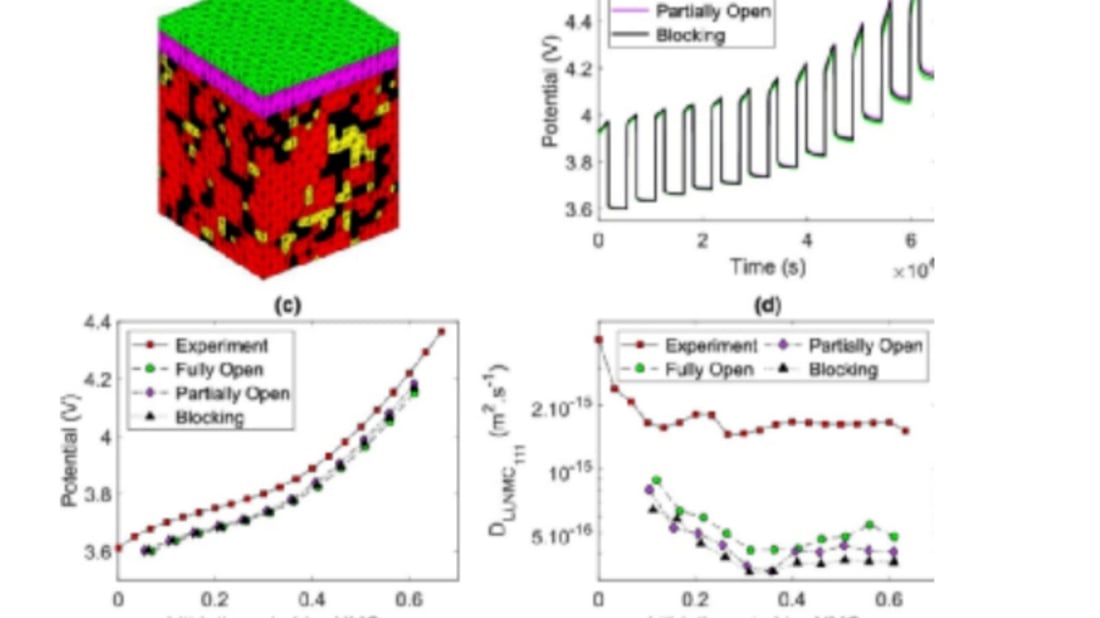
To predict comprehensive electrochemical performance, electrode components are simulated using Coarse-grained Molecular Dynamics (CGMD). Through this approach, mesostructures formed during slurry preparation and drying stages help model the overall electrode. Maintaining computational efficiency involves representing AM particles as spheres with a Gaussian-derived size distribution. The carbon colloidal phase impacts model consistency during simulations.
Simulating the Electrode's 3D Microstructure
Mesostructural data are further processed using COMSOL multiphysics software to predict electrochemical behavior, incorporating open-circuit voltage trends and diffusion coefficients from GITT experiments. By comprehensively integrating the effect of carbon colloidal phase, researchers test three scenarios: (1) Blocking CBD—assuming total Li+ blockage, (2) Partially open CBD—featuring micropores to permit some Li+ transmission at reduced diffusion levels, and (3) Fully open CBD—facilitating unobstructed lithium diffusion comparable to the general electrolyte.
Simulation Findings and Effects
According to the simulations, all scenarios yield similar OCV curve shapes yet lower than empirical data. The effect of carbon colloidal phase emphasizes diffusion coefficient variances: fully open CBDs demonstrate higher diffusion coefficients compared to partially open and blocked configurations. Uneven lithium concentration within larger, isolated NMC particles highlights the effect of carbon colloidal phase further.
Concluding Observations on Diffusion
Elevated blocking traits within the carbon colloidal phase restrict lithium delivery, consequently diminishing the diffusion coefficient D. Hence, the microstructure intricately influences the GITT-generated diffusion coefficients, underscoring the effect of carbon colloidal phase as a critical factor in developing and optimizing lithium-ion battery performance.