Breakthrough in Ultra-Low Temperature FAN electrolyte Technology
Introduction to FAN Electrolyte
Professor Fan Xiulin's recent publication in Nature has introduced a groundbreaking ultra-low temperature electrolyte known as FAN, composed of 1.3M LiFSI/FAN. This advanced electrolyte exhibits impressive conductivity levels of 40.3mS/cm at room temperature and 11.9mS/cm at -70°C. The FAN electrolyte operates effectively in high-energy density lithium-ion batteries, supporting 4.5V NCM811/Gr pouch cells with a surface capacity of 2.85mAh/cm2 over a broad temperature range from -70°C to 60°C.
Unprecedented Performance at Low Temperatures
The FAN electrolyte is capable of rapid charging and discharging within 10 minutes, underpinned by the "ligand channel promoted transport" mechanism. It offers a capacity retention rate of 96% at -35°C and 80% at -60°C, maintaining performance for up to 350 cycles. Traditional EC/EMC-based electrolytes fail under low temperature conditions, making the FAN electrolyte a milestone in the field.
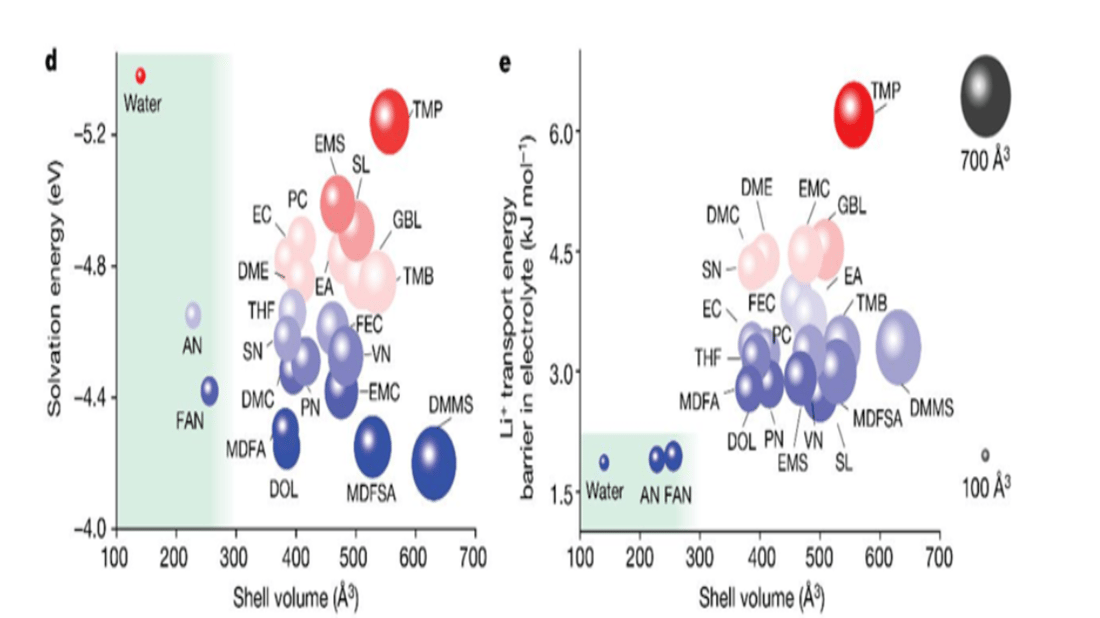
Development and Selection Process
Developing this extraordinary electrolyte required meticulous screening of 23 possible solvents, with analysis focusing on lithium ion solvation energy, solvation sheath volume, and transport energy barriers. Ultimately, water (H2O), acetonitrile (AN), and fluoroacetonitrile (FAN) were selected. FAN emerged as the most viable solvent, capable of forming a stable solid electrolyte interface (SEI) under demanding conditions.
Scientific Validation and Reaction Mechanism
Nitrile additives enhance lithium-ion batteries’ high-temperature performance and thermal stability by forming partially coordinated compounds, reducing parasitic reactions. Acetonitrile forms complexes with lithium through lone pair electrons, which is problematic. However, the electron-withdrawing fluorine in FAN diminishes the lone pair's ability to form coordination compounds with lithium, offering enhanced stability as an electrolyte.
The Future of Electrolyte Innovation
The FAN electrolyte exemplifies how understanding coordination bonds and electron cloud theory can lead to breakthroughs in battery technology. By modifying coordination between graphite and Li+, the theoretical capacity of graphite could potentially exceed current expectations. The advancements in electrolyte chemistry are laying the foundation for more efficient and durable lithium-ion batteries across varied temperatures and conditions.