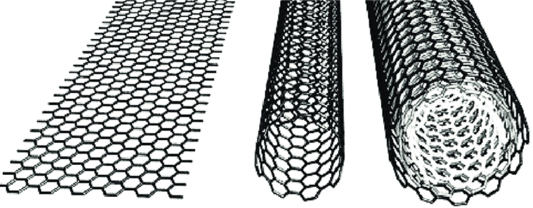
Carbon nanotubes (CNTS) are perfect 1D cylinders composed of one (single-walled carbon nanotube, SWCNT) or multiple graphene sheets (multi-walled carbon nanotube, MWCNT) rolled up and have become the focus of research due to their unique geometry and excellent properties. Both experimental and theoretical analyses show that single-walled carbon nanotubes (SWCNT) and multi-walled carbon nanotubes (MWCNT) have high mechanical strength, high aspect ratio (> 1000), high thermal conductivity, high melting point and low density (1.2-2.6 g/cc). In addition, CNT has a larger surface area (approximately 1000 m²/g), showing excellent hydrogen storage capacity, significant biological properties, and higher corrosion resistance. CNTS also have unique electrical conductivity, and depending on their structure (diameter and helicity), they can exhibit the electronic properties of metals or semiconductors. It is precisely because of its excellent physical and chemical properties that carbon nanotubes have initially blossomed in the field of new materials, gradually realizing industrialization, and are also widely used in the lithium battery industry, attracting more and more attention from the industry.Domestic carbon nanotubes as a conductive agent used in power batteries, its market with the rapid growth of the power battery industry, the scale has reached billions of yuan per year, there are a number of domestic and foreign companies and listed companies have been involved in the field of carbon nanotubes conductive, such as Dow Technology, German nano, Cabot and other companies. With the expansion of the application scale of new technologies such as high-nickel positive lithium batteries, silicon-based negative batteries and solid-state batteries, the market for carbon nanotubes will also grow explosively, and it will become an annual market of 10 billion in the field of lithium batteries alone.In addition to excellent electrical conductivity, carbon nanotubes also have a huge potential market for development such as ultra-high mechanical and thermal properties. The 1996 Nobel Prize winner in Chemistry, the Honourable Richard E. Smalley (the discoverer of fullerenes), once said: "Carbon nanotubes are the strongest, toughest and hardest molecules that can be made, and are the best molecular conductors of heat and electricity." It sounds like this is the good prediction and desire of scientists, but this is the original intention of Juyuan Material Technology (Zunyi) Co., Ltd. to intervene in the carbon nanotube industry, and it is also the goal of industrialization. The application of lithium battery conductive agent is only the "tip of the iceberg" of the industrialization of carbon nanotubes, and it is the first step for capital and traditional chemical new material enterprises to understand carbon nanotubes. Carbon nanotubes in the touch screen, transistors, biomedical, solar photovoltaic cells, tires, fuel cells, drug delivery, hydrogen storage, polymer materials, capacitors, composite materials and other fields will have a broader market, will continue to break through more than 10 billion of the market, become a 100 billion market industry.The huge carbon nanotube application market requires sufficient carbon nanotube production capacity to support and a sustainable supply of low-cost high-quality carbon nanotubes in order to open a huge downstream potential application market. The early tonnage production of carbon nanotubes was achieved by fixed bed/moving bed equipment, and the continuous addition of catalysts and hydrocarbon raw materials and the continuous output of carbon nanotube products in a horizontally placed tube furnace through a sophisticated device can realize the production of the first generation of carbon nanotube plants with tons to tens of tons per year in a single device. Up to now, there is still some market space for some special types of carbon nanotubes, or some special raw materials for carbon nanotube production, using fixed bed/moving bed first-generation carbon nanotube factories.In order to achieve larger scale carbon nanotube production, the second-generation carbon nanotube factory with fluidized bed reactor solves the larger scale production problem to a certain extent, and the annual production capacity of a single reactor can reach 100 tons to hundreds of tons. At present, the large-scale production of carbon nanotubes in China, many production companies are basically based on fluidized bed reactors. With the emergence of larger production requirements, fluidized bed reactors have the following characteristics: 1. They cannot be scaled up; 2, easy carbon deposition, coking plugging reactor; 3, the boiler shutdown and cleaning of the reactor is difficult, the time-consuming period is long and other problems, which seriously restricts the expansion of the single production capacity of the fluidized bed reactor. At present, the inner diameter of the fluidized bed reactor in the industry is basically 500mm\600mm\800mm, and it is difficult to further expand.